Rubin Lab
UCSC
Mechanisms of Multisite Cdk Phosphorylation
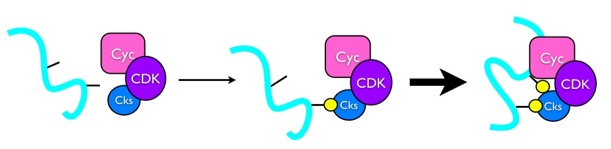
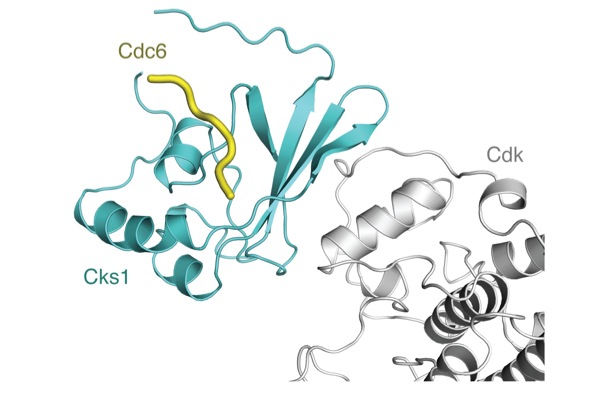
Protein phosphorylation is central to cell cycle signal integration and phosphorylation of regulatory proteins on multiple sites generates intricate responses and signaling properties. How cells use multisite phosphorylation to produce diverse outputs is a critical question as cancer cells have invariably lost cell cycle control, but the enzymatic mechanisms that tune multisite phosphorylation are poorly understood. In collaboration with the Kellogg laboratory in MCD Biology, we study the Cks protein and its role in stimulating multisite phosphorylation by the Cyclin-dependent kinase (Cdk) cell cycle control complex. We are currently testing a model in which Cks directs Cdk to specific, primed substrates to enhance kinetics and signal output. These investigations include a novel mass spectrometry assay for site-specific phosphorylation kinetics and x-ray crystallography to determine the origins of Cks-Cdk-substrate specificity. This project is funded by the American Cancer Society.